

Introduction
Chemiluminescence Immunoassay (CLIA) is the mainstream testing method adopted by clinical in vitro diagnosis, which is widely used in disease diagnosis of tumors, hormones, cardiovascular diseases, infection and so on. Compared with other immune detection methods, CLIA has advantages such as high sensitivity, wide detection range, short detection time, and high degree of automation.
The development of CLIA diagnostic kits involves the development of antigen and antibody reagents, the conjugation of reagents, the establishment of immune detection methods, the determination of production solutions, instrumental adaptation, and performance evaluation. HKIG has many years of experience in the research and development field of in vitro diagnostic reagents. The company has developed a variety of CLIA kits for diagnostic purpose, and helps partners to successfully obtain medical device registration.Introduction
HKIG provides customers with a comprehensive “one-stop” CLIA diagnostic kit development solution, providing customers with all technical supports in the development of CLIA diagnostic kit.
Example
For a tumor marker detection, a paired monoclonal antibody has been developed, and the CLIA kit was further constructed. This kit can detect the target marker with high sensitivity and high specificity.
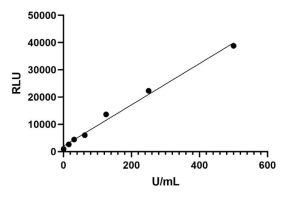
R2>0.99,LoB < 0.5U/mL
