Introduction
Immunodetection technology has high sensitivity, strong specificity, stability and reliability, and is the mainstream technology of medical in vitro diagnosis, which is widely accepted and used in clinical practice. Obtaining the “Prototype” of immunodiagnostic products is the primary condition and key link for the industrialization and clinical application of innovative academic research results.
Service Feature
HKIG has nearly 20 years of experience in the development of immunodiagnostic products. According to the different characteristics of biomarkers and detection needs, HKIG develops personalized “prototypes” of immunodiagnostic products for customers, which greatly shortens the time from the laboratory stage to the clinical trial and registration stage, and helps our customers successfully realize the industrialization of innovative research results.
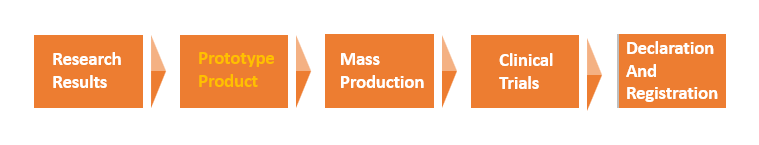
Service Introduction
What do you need to provide? You only need to provide the innovative marker name or sequence, structural information, detection requirements, technical performance requirements.
What will we do? We will carry out immunoassay protocol design (based on one or several different technology platforms such as enzyme immunoassay, chemiluminescence, flow fluorescence, immunochromatography, etc.), original antigen and antibody raw materials and reagents development, detection method establishment, product prototype construction and performance evaluation.
What do you get? The “prototype” of immunodiagnostic products to meet your testing needs, independent and controllable core raw materials, detailed and complete production process plan, product manuals and other product prototype development all technical support.
Service Case
Certain cytokines are important effectors of inflammatory response and pathogenic factors of a variety of autoimmune diseases. Entrusted by the client, HKIG developed the sandwich paired antibody detection reagent, antigen quality control material, and constructed the high sensitivity ELISA detection kit. The kit has a sensitivity of pg/mL to meet customers’ clinical testing needs (EBioMedicine. 2023; 92:104607).

Figure: Kit standard curve(R2>0.99)


